INDICATION
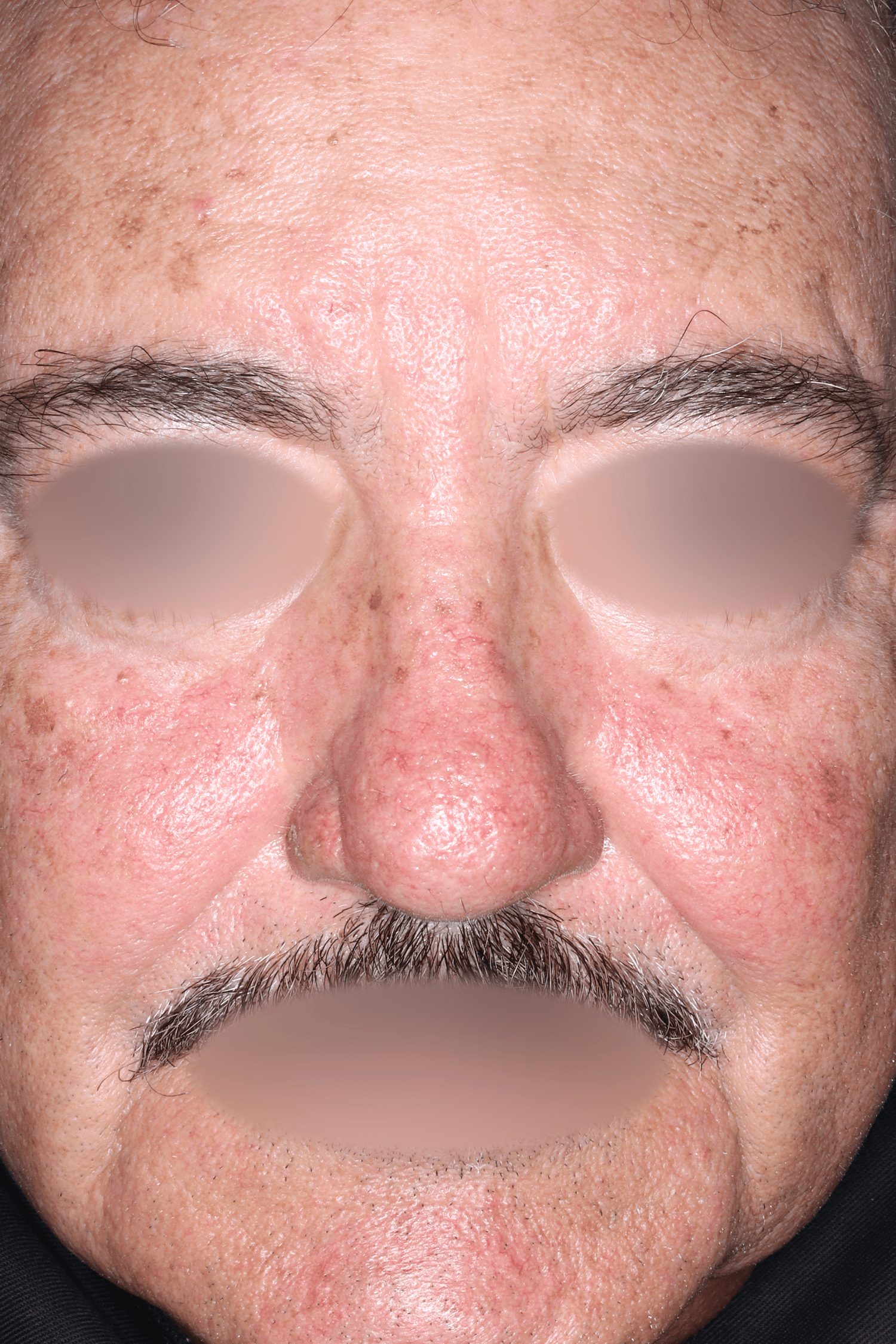
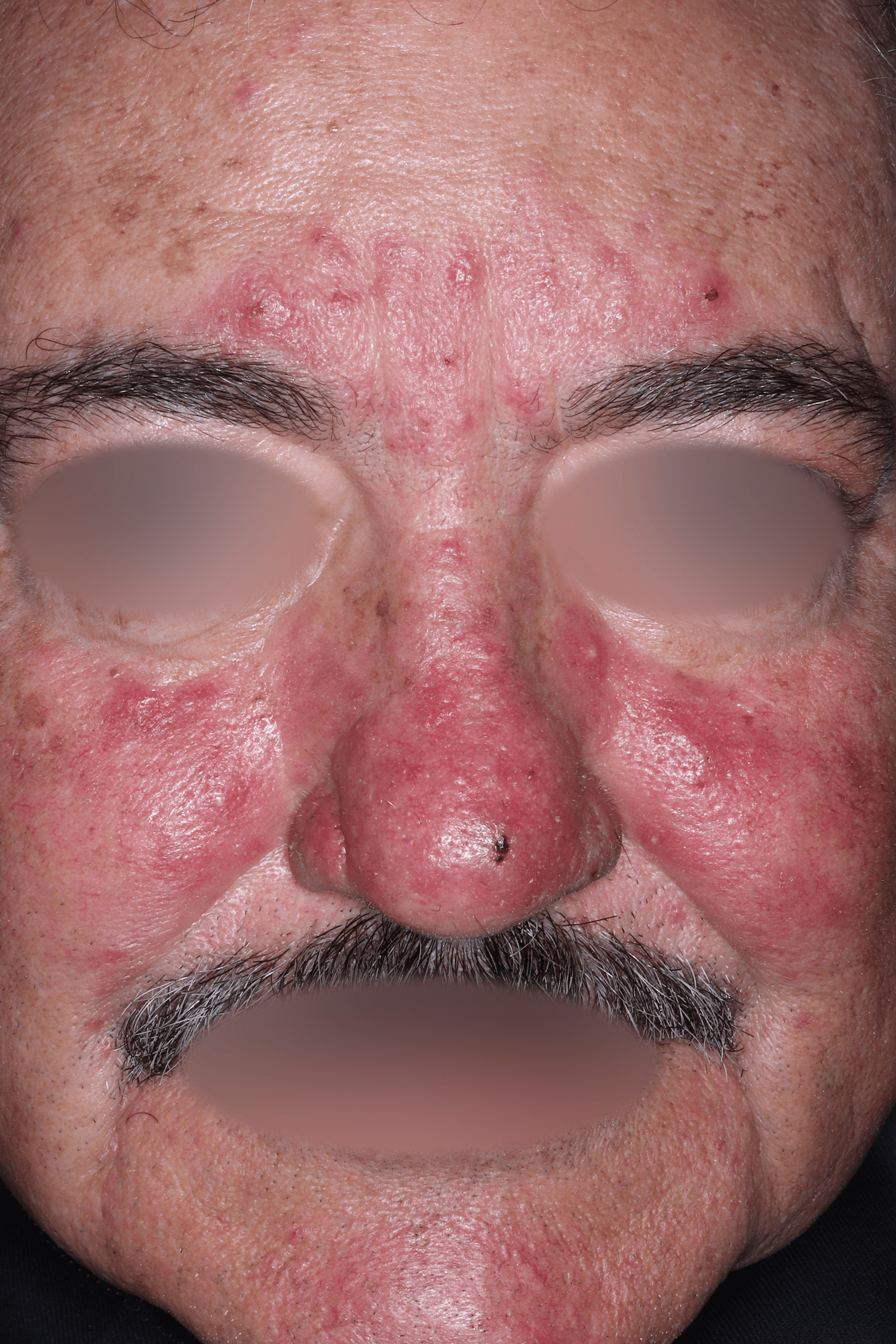
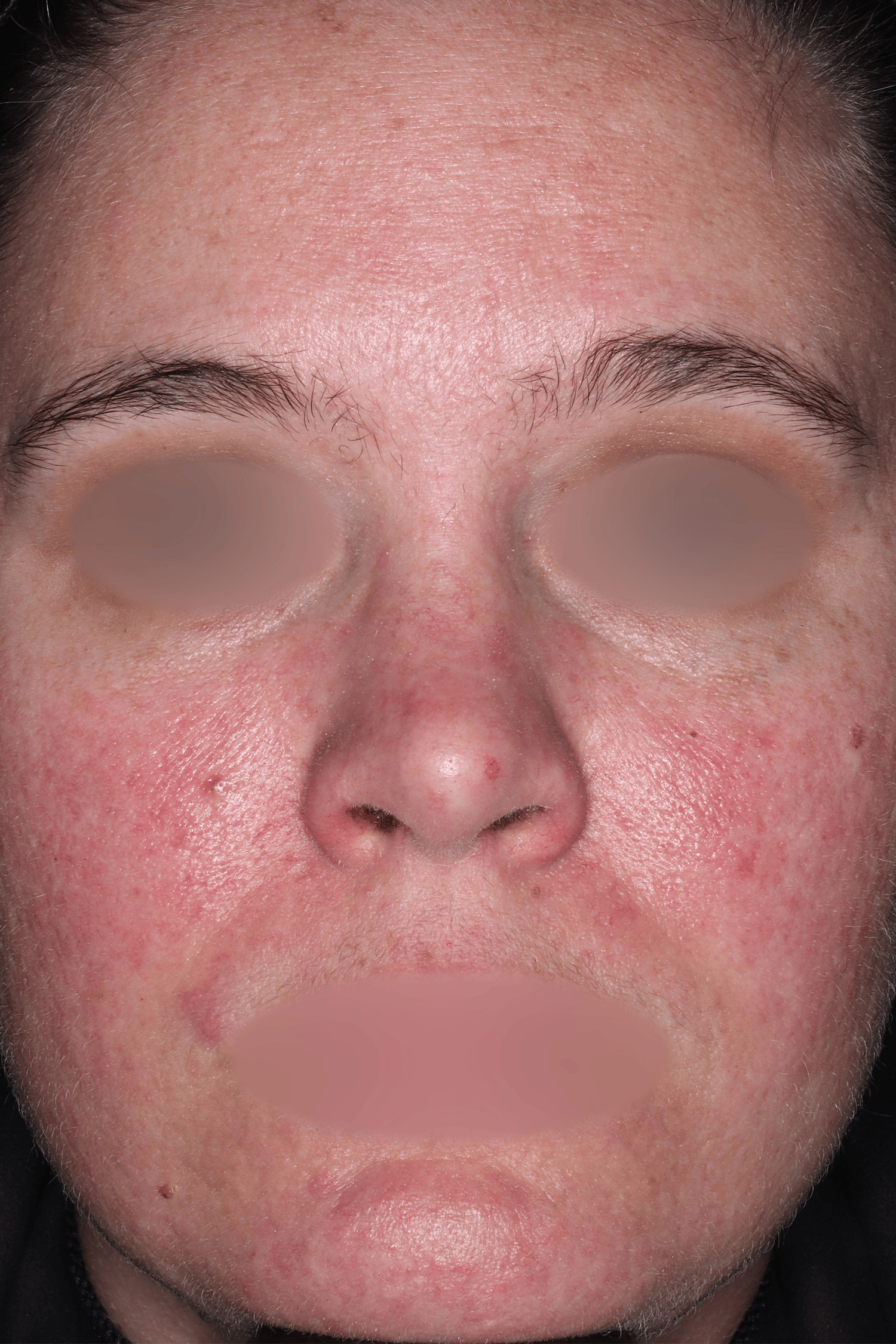
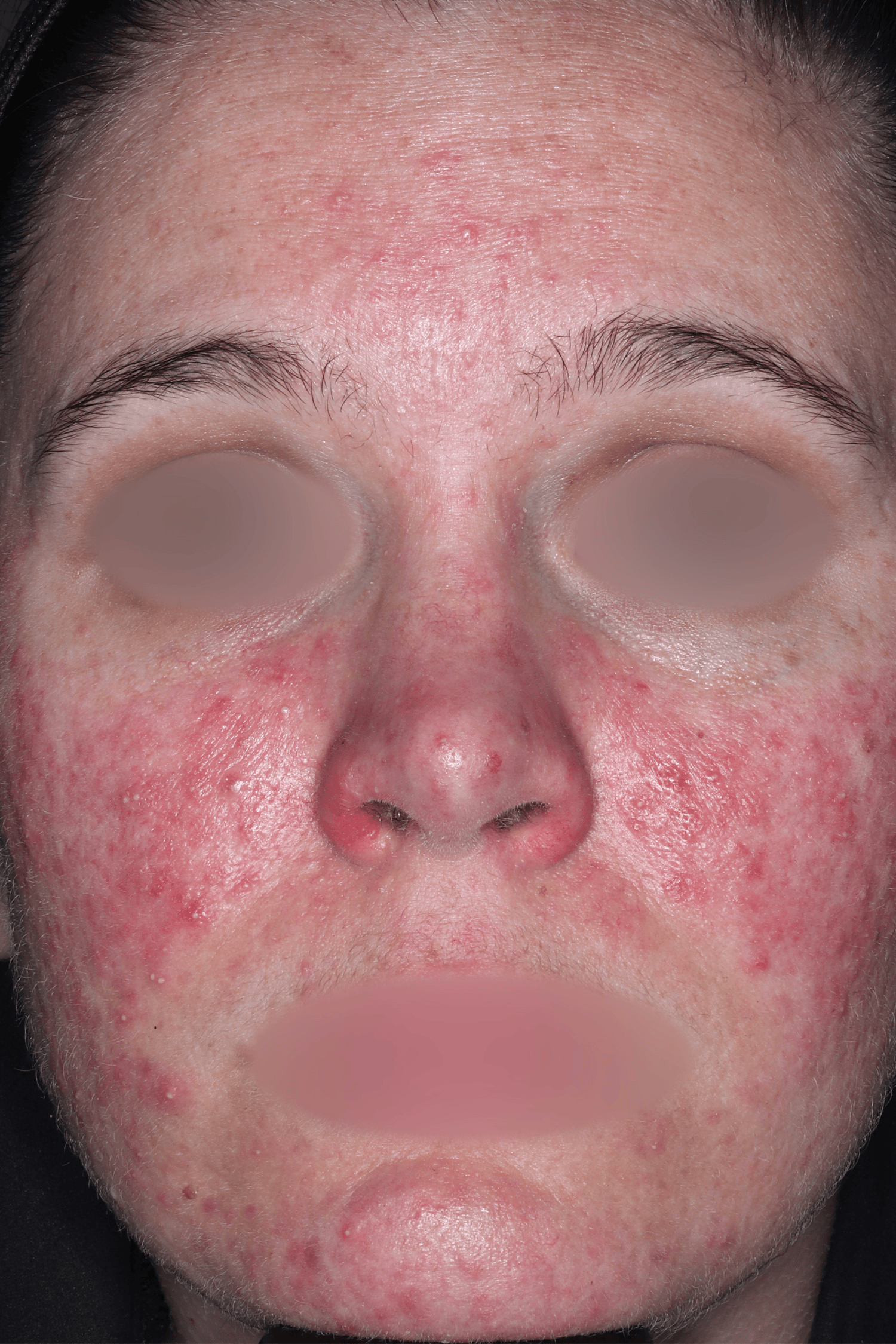
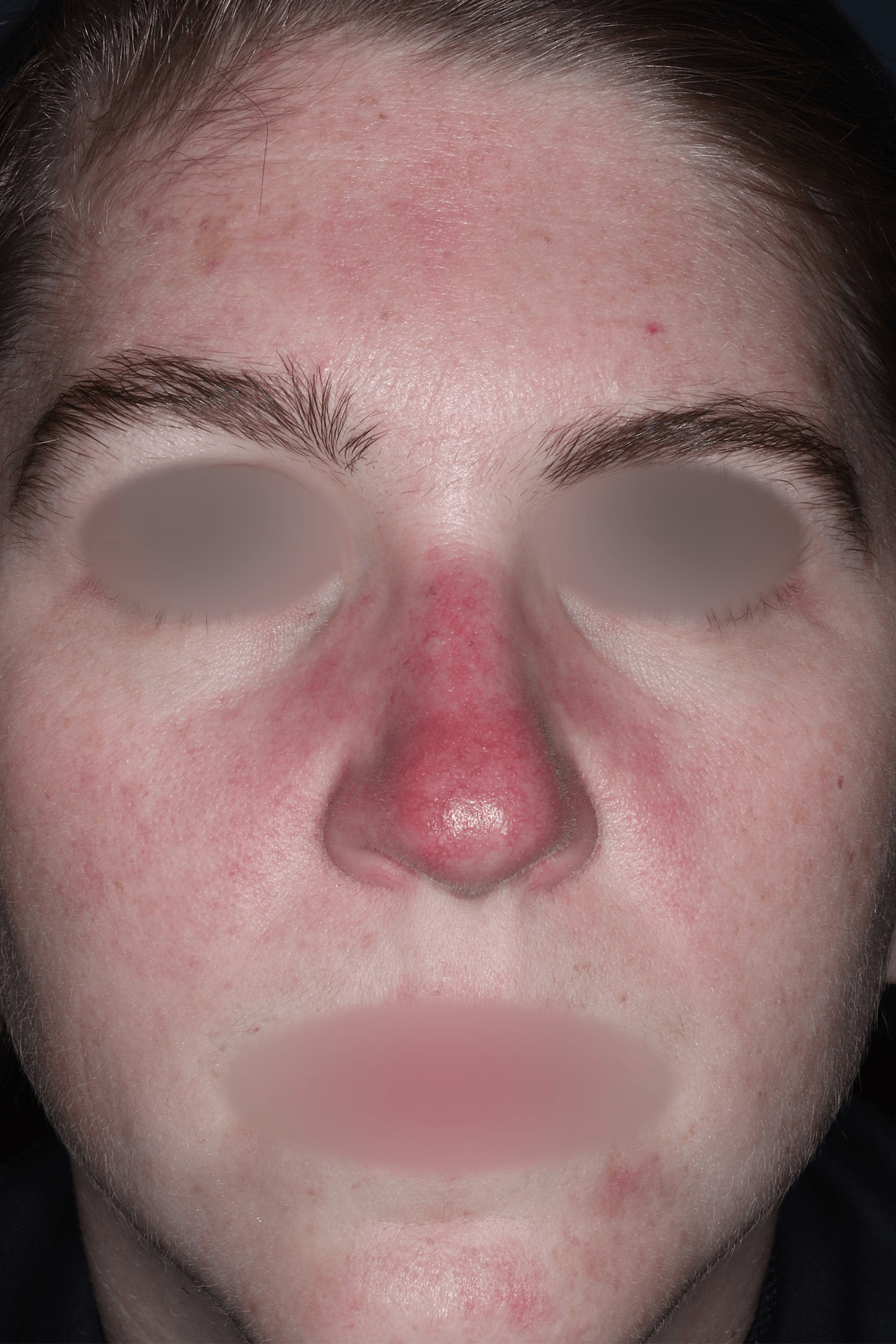
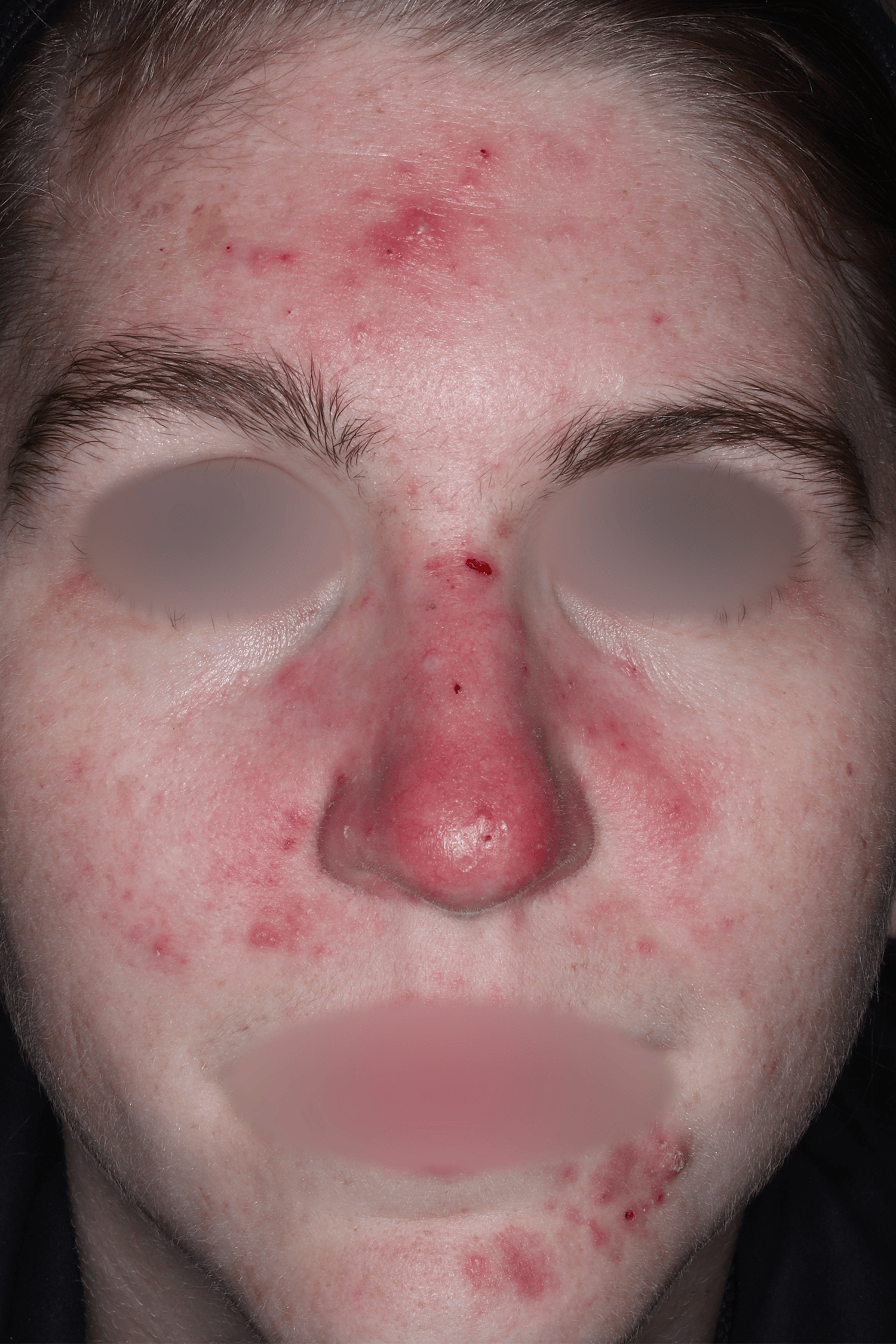
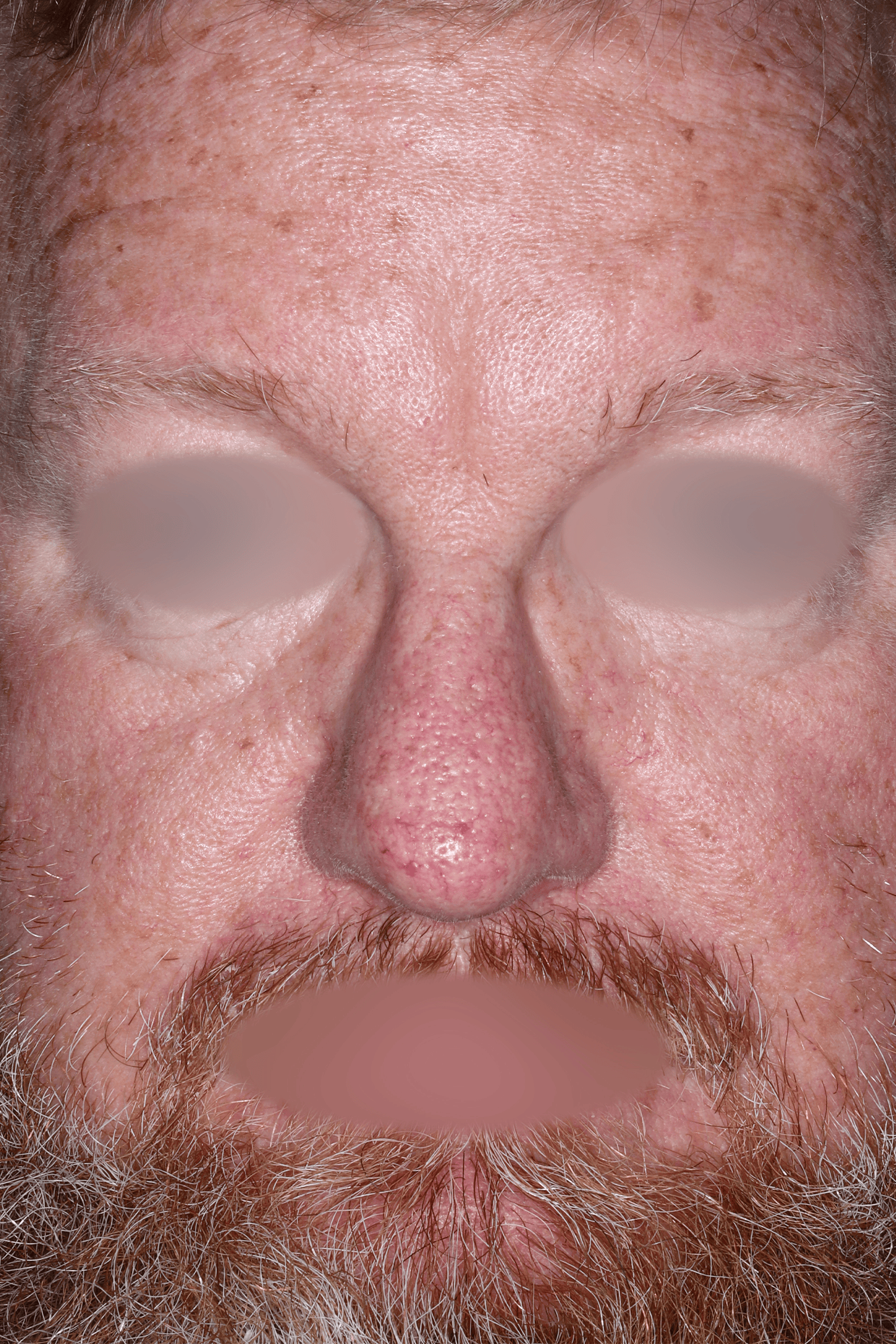
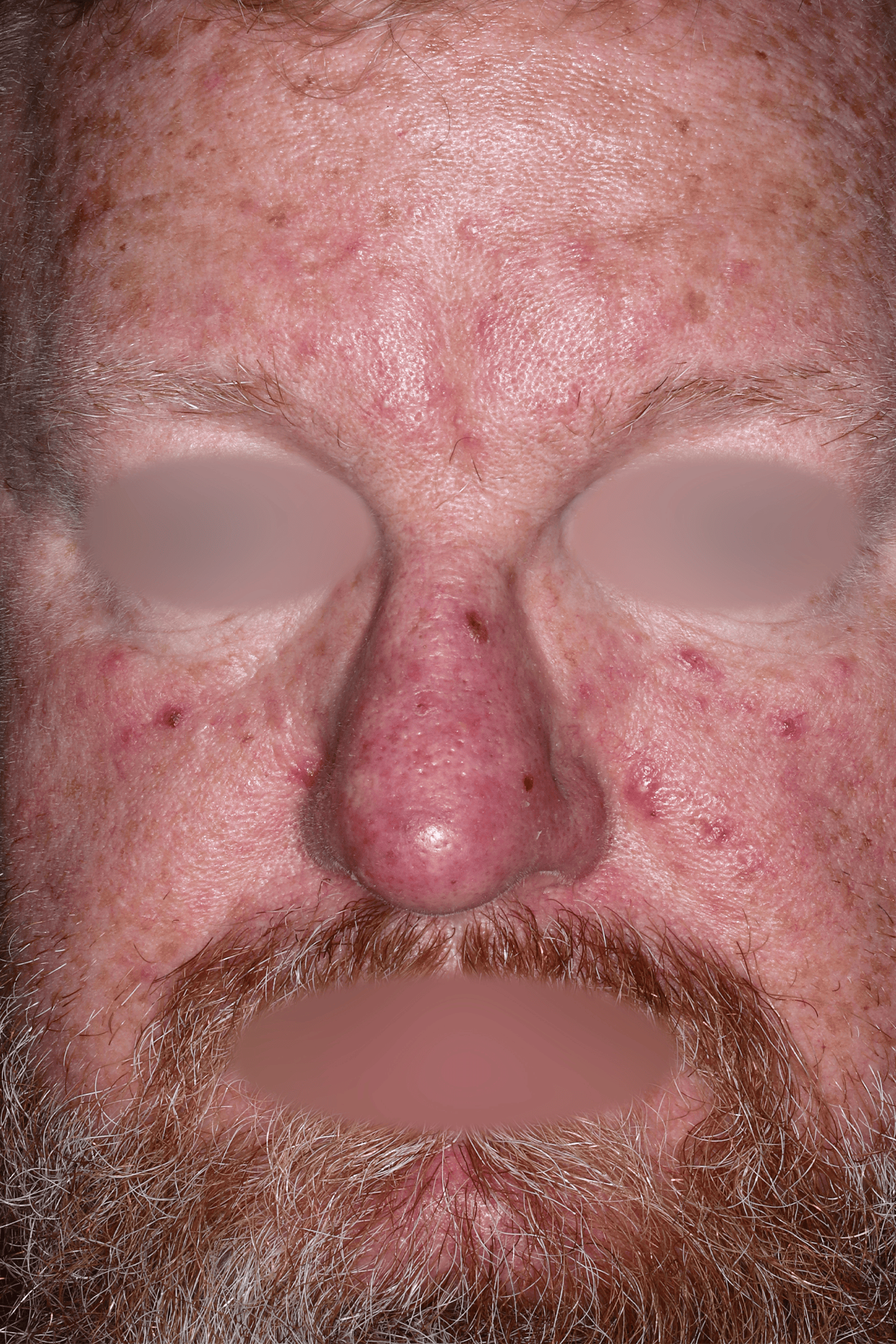
EMROSI is a prescription medicine used to treat adults with pimples and bumps (inflammatory lesions) caused by a condition called rosacea.
EMROSI should not be used for the treatment of infections. It is not known if EMROSI is safe and effective in children.
IMPORTANT SAFETY INFORMATION
- Do not take EMROSI if you are allergic to any tetracycline medicines.
- Before using EMROSI, tell your healthcare provider about all the other medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. EMROSI and other medicines may affect each other and cause serious side effects.
- Do not use EMROSI if you are pregnant, may become pregnant or are nursing. If you become pregnant while using EMROSI, talk to your doctor. Tetracycline medicine when taken by mouth during pregnancy, infancy and/or childhood up to the age of 8 years may permanently discolor teeth (yellow-gray-brown) and may slow the growth of bones.
- Protect your skin from the sun while using EMROSI. Avoid sunlight or artificial sunlight such as sunlamps or tanning beds. Stop using EMROSI if you get a sunburn.
- When taken by mouth, minocycline may cause feelings of lightheadedness, dizziness or a spinning feeling. You should not drive or operate dangerous machinery if you have these symptoms.
EMROSI is an extended-release capsule that contains minocycline, a tetracycline medicine. Tetracyclines, when taken by mouth, may cause serious side effects, including: serious skin or allergic reactions which can cause skin redness, rash, hives, sores in your mouth, or your skin blisters and peels; swelling of your face, eyes, lips, tongue, or throat; trouble swallowing or breathing; blood in your urine; fever, yellowing of the skin or the whites of your eyes (jaundice), dark colored urine; pain on the right side of the stomach area (abdominal pain); chest pain or abnormal heartbeats; swelling in your legs, ankles, and feet; diarrhea which may be caused by an infection that can cause watery or bloody stools; loss of appetite; tiredness; unexplained bleeding or bleeding more easily than normal; confusion; sleepiness; vision changes, including blurred vision, double vision, or permanent vision loss; unusual headaches; fever; rash; joint pain; body weakness. Call your doctor right away if these side effects occur.
The most common side effects of EMROSI include stomach upset or burning (dyspepsia) after eating or drinking. Call your doctor for medical advice about side effects.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report side effects to Journey Medical Corporation at 1‑855‑531‑1859.